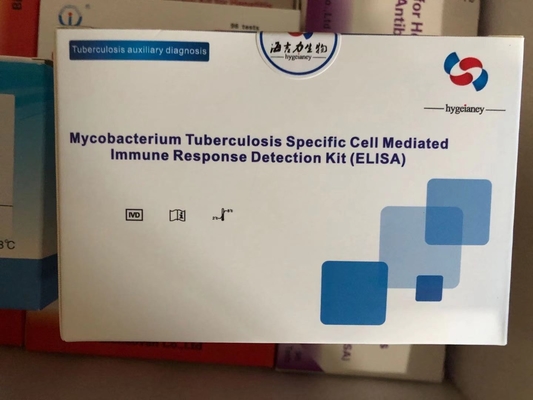
TB-IGRA ELISA TEST KIT
1. Principle
TB-IGRA is an in vitro diagnostic test that detects cell-mediated immune responses to Mycobacterium tuberculosis (MTB) infection by measuring interferon-gamma (IFN-γ) released by sensitized T cells upon stimulation with MTB-specific antigens (e.g., ESAT-6, CFP-10, and TB7.7).
| Product details |
Description |
| Delivery |
Within 48 hours |
| Packaging Specifications |
8 x 12 strips, 96 wells |
| Country Of Origin |
China |
| Manufacturer |
18 months |
| Preservation method |
2℃-8℃ |
| Specimen |
Whole blood |
| Assification |
class1 |
| Type |
Elisa Test Kit |

2. Key Features
3. Sample Requirements
-
Sample Type: Fresh peripheral whole blood (heparinized).
-
Volume: Usually 1–3 mL per test (varies by kit manufacturer).
-
Storage: Process within 8–32 hours (depending on kit specifications).
4. Assay Procedure
-
Stimulation: Incubate blood with MTB antigens and controls (negative/mitogen control).
-
IFN-γ Detection:
-
ELISPOT-based (e.g., T-SPOT.TB): Count IFN-γ-secreting T cells as spots.
-
ELISA-based (e.g., QuantiFERON-TB Gold): Measure IFN-γ concentration via immunoassay.
-
Interpretation:
5. Performance Metrics
-
Sensitivity: >90% for active TB, ~80% for LTBI.
-
Specificity: >95% (unaffected by BCG vaccination).
-
Cross-reactivity: Minimal with non-tuberculous mycobacteria (NTM).
6. Indications
-
Screening for LTBI in high-risk groups (e.g., HIV+, immunosuppressed patients, close contacts).
-
Supplemental test for active TB diagnosis (must correlate with clinical symptoms and other tests).
7. Advantages Over TST
-
No cross-reactivity with BCG.
-
Single-visit testing (no return for reading).
-
Objective, lab-based results.
8. Limitations
-
Cannot distinguish active TB from LTBI.
-
Requires specialized lab equipment.
-
Higher cost than TST.
-
Potential false negatives in immunocompromised patients.
9. Compatible Kits (Examples)
10. Regulatory Status
In Vitro Diagnostic
Use Only
This kit contains reagents sufficient for testing of maximum of 28 specimens in a test run. Stimulation Components:
1. Negative Control (Grey lid)
2. TB Antigen, lyophilized (Purple lid)
3. TB Antigen Diluent (Purple lid)
4. Positive Control (Red lid) 28tests 28tests 0.9mL 28tests
ELISA Components
1. Microplate Strips
2. Human IFN-γ Standard, lyophilised (Blue lid)
3. Diluent
4. Biotin Labeled Antibody (Yellow cap) 12x 8 well 2 x vial 1 x 40mL 1 x 6mL
5. Enzyme Conjugate 100X Concentrate (Brown bottle) 1 x 130μL
6. Wash Buffer 10X Concentrate 1 x 40mL
7. Chromogen Solution A
8. Chromogen Solution B
9. Stopping Solution 1 x 6mL 1 x 6mL 1 x 6mL
PRINCIPLE OF THE TEST
This kit uses the principle of IGRA combined with enzyme linked immunosorbent assay (ELISA) to
measure specific antigen mediated immune response strength. The antigens selected for stimulation is
specific fragments that pathogenic Mycobacterium tuberculosis have, but BCG vaccine and other
Mycobacterium do not have. The patients infected with Mycobacterium tuberculosis have specific T
lymphocytes which can identify these antigens and T lymphocytes will be stimulated to proliferate and
release cytokines such as IFN-γ. This kit uses ELISA method for quantitative detection the specific IFN-γ
released by cells to determine Mycobacterium tuberculosis infection.










 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!