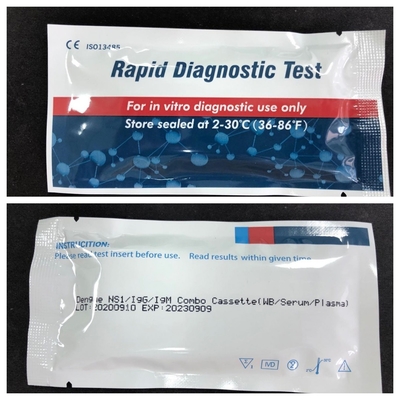
Product Description:
The Dengue IgG/IgM3.0 Rapid Test is a lateral flow immunoassay for the simultaneous detection and differentiation of IgG anti–dengue virus and IgM anti-dengue virus in human serum or plasma. It is intended to be used by the professionals as a screening test and as an aid in the diagnosis of infection with dengue viruses. Any confirmed with alternative testing method(s).
Applications:
Dengue reagents are mainly used to detect dengue virus or its antibodies, and are generally divided into two categories:
1. **Virus detection reagents:** Used to directly detect the presence of the virus. Common methods include PCR (polymerase chain reaction) and antigen detection. PCR can detect the presence of viral RNA, while antigen detection can detect specific proteins of the virus in the blood.
2. **Antibody detection reagents:** Used to detect the body's immune response to dengue virus, usually through serological testing. These reagents detect IgM and IgG antibodies in serum and can help diagnose whether dengue virus has been infected or is in the process of infection.
The application of dengue reagents is mainly used in the following aspects:
- **Early diagnosis:** Rapid detection methods such as antigen detection can help diagnose dengue fever early, especially in epidemiological surveys and outbreak management.
- **Epidemic monitoring:** By monitoring the antibody levels in the population, the spread and outbreak trends of dengue fever can be evaluated, guiding the formulation and adjustment of public health intervention measures.
- **Immunology research:** Research on the immune response to dengue virus, including the production and protective effect of antibodies, is of great significance to vaccine development and immune strategy formulation.
In general, dengue reagents play an important role in the prevention, diagnosis and control of dengue fever, especially in epidemiological surveys, epidemic monitoring and vaccine development.
Precautions:
The precautions when using dengue fever reagents mainly include the following points:
1. **Correct operation:** The reagents must be operated according to the instructions provided by the manufacturer to ensure the correct use and interpretation of the reagent results.
2. **Quality control:** Before each use of the reagent, a quality control test must be performed to confirm the accuracy and reliability of the reagent.
3. **Avoid cross contamination:** During the use of the reagent, the disinfection and cleaning operating procedures must be strictly followed to avoid cross contamination and reagent contamination.
4. **Storage conditions:** The storage conditions of the reagents should be carried out in accordance with the manufacturer's recommendations, usually stored within the specified temperature range and under light-proof conditions to maintain the stability and effectiveness of the reagents.
5. **Clinical application with caution:** In clinical applications, the results of the reagents should be used as an auxiliary basis for diagnosis, and comprehensive analysis should be conducted in combination with clinical symptoms and other laboratory test results.
6. **Strict compliance with laws and regulations:** Laboratories and medical institutions using dengue fever reagents must strictly follow relevant laws and regulations and laboratory standards to ensure safety and compliance.
In short, the correct and cautious use of dengue fever reagents is the key to ensuring the accuracy and safety of test results.
FAQ:
Q: What is the brand name of the Elisa Test Kit?
A: The brand name of the Elisa Test Kit is Biovantion.
Q: What is the model number of the Elisa Test Kit?
A: The model number of the Elisa Test Kit is BVTE0097.
Q: Where is the Elisa Test Kit manufactured?
A: The Elisa Test Kit is manufactured in China.
Q: What certification does the Elisa Test Kit have?
A: The Elisa Test Kit has ISO13485 certification.
Q: What is the minimum order quantity for the Elisa Test Kit?
A: The minimum order quantity for the Elisa Test Kit is 10.
Q: What is the price of the Elisa Test Kit?
A: The price of the Elisa Test Kit is negotiable.
Q: What are the packaging details for the Elisa Test Kit?
A: The packaging details for the Elisa Test Kit are carton.
Q: What is the delivery time for the Elisa Test Kit?
A: The delivery time for the Elisa Test Kit is 7-15 days.
Q: What are the payment terms for the Elisa Test Kit?
A: The payment terms for the Elisa Test Kit are TT 100% payment.
Q: What is the supply ability of the Elisa Test Kit?
A: The supply ability of the Elisa Test Kit is 100000.





 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!