Helicobacter (H.P) antibody (IgG) ELISA kit
For in vitro diagnostic and professional use only. 96TESTS/KIT
INTENDED USE
This kit is used for qualitative detection of Helicobacter pylori IgG antibodies in human serum/plasma,which is based on Enzyme Linked Immunosorbent assay.This test kit provides only a preliminary test result for H.P infection as a clinically-assisted diagnosis. Testing is only limited to professional laboratories.
| Product details |
Description |
| Delivery |
Within 48 hours |
| Packaging Specifications |
8 x 12 strips, 96 wells |
| Country Of Origin |
China |
| Manufacturer |
18 months |
| Preservation method |
2℃-8℃ |
| Specimen |
Whole blood |
| Assification |
class1 |
| Type |
HAV IgM Elisa Test Kit
|
PRINCIPLE
Indirect method, total duration of assay: 75 minutes.
The DiaSino H.Pylori IgG ELISA employs solid phase, indirect ELISA method for detection of antibodies to H.Pylori IgG in two-step incubation procedure.
Polystyrene microwell strips are pre-coated with highly immunoreactive human H.Pylori antigens. During the first incubation step, anti-H.Pylori IgG specific antibodies, if present, will be bound to the solid phase pre-coated H.Pylori antigens. The wells are washed to remove unbound serum proteins, and rabbit anti-human IgG antibodies (anti-IgG) conjugated to the enzyme horseradish peroxidase (HRP-Conjugate) are added. During the second incubation step, these HRP-conjugated antibodies will be bound to any antigen-antibody(IgG) complexes previously formed and the unbound HRP-conjugate is then removed by washing. Chromogen solutions containing Tetramethylbenzidine (TMB) and urea peroxide are added to the wells and in presence of the antigen-antibody-anti-IgG (HRP) immunocomplex, the colorless Chromogens are hydrolyzed by the bound HRP conjugate to a bluecolored product. The blue color turns yellow after stopping the reaction with sulfuric acid.
SUMMARY
Helicobacter pylori is one of the most common bacterial infections in humans, affecting nearly 50% of the global population. H.pylori has been associated with the development of serious upper gastrointestinal (GI) conditions including chronic gastritis, peptic ulcer disease, gastric cancer,and mucosa-associated lymphoid tissue (MALT).
A serological test has been the first choice for the detection of H. pylori infection because it is easy to perform compared to the more invasive diagnostic tests. A positive serologic test indicates the presence of H. pylori antibodies that confirms both a possibility for past infection or potential current infection.

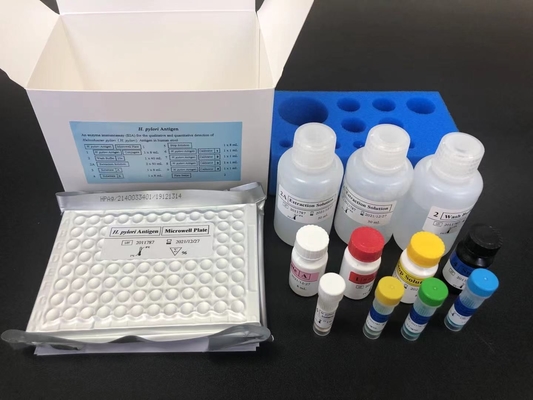
Materials provided with the kit:
| Item |
Components |
|
| 1 |
H.Pylori antigen Microplate |
1plate |
96 wells |
| 2 |
HRP-Anti-Human IgG Conjugate |
1vial |
12mL |
| 3 |
H.Pylori IgG Positive Control |
1 vial |
1mL |
| 4 |
H.Pylori IgG Negative Control |
1 vial |
1mL |
| 5 |
Sample Diluent |
1 vial |
12mL |
| 6 |
Wash Buffer(20X) |
1 vial |
40mL |
| 7 |
Substrate A |
1 vial |
6.0mL |
| 8 |
Substrate B |
1 vial |
6.0mL |
| 9 |
Stop Solution |
1 vial |
6.0mL |
| 10 |
CARDBOARD PLATE COVER |
3 sheets |
| 11 |
Product Insert |
1 |
| Others 1 x Resealable Bag |
Materials and reagents required but not provided in the kit
1. Pipette capable of delivering5µL, 50 µL and 100 µL volumes
TEST PRINCIPLE
Hepatitis A virus (HAV) is a kind of RNA virus, which belongs to the family of Picornavirus. HAV showed a spherical particles shape with 27 nm in diameter, icosahedral symmetry consisted of 32 shell particles, containing linear single-strand RNA. Hepatitis A is a kind of intestinal infectious disease caused by hepatitis A virus (HAV) with a worldwide distribution. Children and adolescents are most likely to be infected, and the peak incidence is in winter and spring. Hepatitis A is the most common type of acute viral hepatitis, mainly spread by fecal-oral route. The incubation period of hepatitis A virus is 15 to 45 days, HAV-IgM antibody can be detected in serum/plasma a short time later after infected, continue to rise very rapidly, peaking in about 2 weeks, decreasing gradually, disappear in 8 weeks. While HAV-IgG antibody appear later than IgM and will persistent for a long time. Detection of HAV- IgM antibody can diagnose HAV infection in early stage for its simple, fast, and high specificity.
This kit uses capture ELISA principle to detect HAV IgM. Purified anti-human IgM antibody is pre-coated on the microplate, the HAV IgM in sample will combine with anti-human IgM first, then combine with enzyme-labeled anti-HAV-HAV antigen complex, and shows blue color in the microplate. This kit is used for the specific detection of HAV IgM antibody in human serum/plasma.
2.Calibrated microplate reader capable of reading at 450 nm with a 630-700 nm reference filter, or reading at 450 nm without a reference filter
3. Absorbent paper for blotting the microwells
4. Timer
5. Distilled or de-ionized water
STORAGE AND STABILITY
Store all components at 2-8°C. Do not freeze. Ensure that the reagents are brought to room temperature before opening. Return all reagents requiring storage at 2-8°C to refrigeration immediately after use. Re-seal the unused microwells in provided resealable bag with desiccant. All reagents are stable through the expiration date printed on the label if not opened. Once opened, the kit is stable for 8 weeks at 2-8°C, or until the labeled expiration date, whichever is earlier.
SPECIMEN COLLECTION AND PREPARATION
1. Serum or plasma should be prepared from a whole blood specimen obtained by acceptable venipuncture technique.
2. This kit is designed for use with serum or plasma specimens without additives only.
3. If a specimen is not tested immediately, refrigerate at 2-8°C for up to 3 days. For storage longer than 3 days, the specimen should be frozen at -20°C. Avoid multiple freeze-thaw cycles. If a specimen is to be shipped, pack in compliance with federal regulations covering the transportation of etiologic agents.
4. Specimens containing precipitates may give inconsistent test results. Clarify such specimens by centrifugation prior to performing the assay.
5. Do not use specimens demonstrating gross lipemia, gross hemolysis or turbidity. Do not use specimens containing sodium azide.
PREPARATION OF THE REAGENTS PRIOR TO ASSAYING
1. Bring all reagents and controls to room temperature (18-28°C).
2. Preparation of working Wash Buffer:
If precipitants are visible, warm up the Wash Buffer (20X) at 37˚C. Dilute concentrated Wash Buffer (20X) at the rate of 1:20 dilution with distilled water.













 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!