Mycobacterium Tuberculosis Specific T Cell Mediated Immune Response Detection Elisa test Kit
Intended use
This kit is an enzyme-linked immunosorbent assay for quantitative detection of Interferon Gamma (IFN-γ) that responds to in-vitro stimulation by Mycobacterium tuberculosis antigens in fresh human whole blood. It is intended for use as an aid in the diagnosis of tuberculosis (TB) infection.
Summary
Tuberculosis is a communicable disease caused by infection with M.tuberculosis (MTB)complex organisms (M. tuberculosis, M. bovis, M. africanum), which typically spread to new hosts via airborne droplet nuclei from patients with respiratory tuberculosis disease. It is one of the most important health threatening problems worldwide. The World Health Organization estimates that one third of the world’s population is infected with M.tuberculosis. Each person carrying latent TB infection (LTBI) has approximately a 10% chance of progressing to active disease. Interferon Gamma Release Assay (IGRA) measure a person’s immune reactivity to M.tuberculosis. Tlymphocytes from most persons that have been infected with M.tuberculosis will release IFN-γ when mixed with special antigens derived from M.tuberculosis. IGRA is whole-blood test that can aid in diagnosing Mycobacterium tuberculosis infection, including both latent tuberculosis infection (LTBI) and tuberculosis (TB) disease.
Technical Parameters:
| Product Name: |
Mycobacterium Tuberculosis Diagnostics
|
| Manufacturer: |
Biovantion |
| Test Type: |
ELISA |
| Sample Type: |
Serum, Plasma |
| Assay Time: |
2-3 Hours |
| Sensitivity: |
High |
| Specificity: |
High |
| Storage Temperature: |
2-8°C |
| Shelf Life: |
18 Months |
| Kit Size: |
96 Tests |
Test principle
This kit uses the principle of IGRA combined with enzyme linked immunosorbent assay (ELISA) to measure specific antigen mediated immune response strength. The antigens selected for stimulation is specific fragments that pathogenic Mycobacterium tuberculosis have, but BCG vaccine and other Mycobacterium do not have. The patients infected with Mycobacterium tuberculosis have specific Tlymphocytes which can identify these antigens and T lymphocytes will be stimulated to proliferate and release cytokines such as IFN-γ. This kit uses ELISA method for quantitative detection the specific IFN-γreleased by cells to determine Mycobacterium tuberculosis infection.
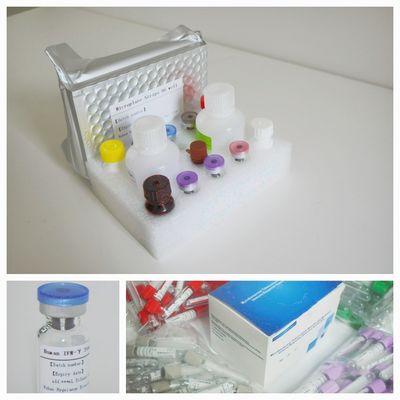
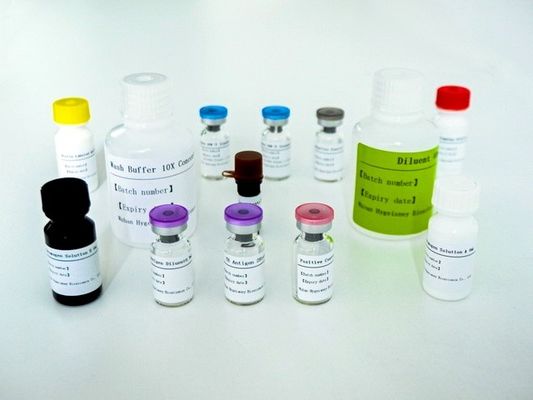
Components
This kit contains reagents sufficient for testing of maximum of 28 specimens in a test run.
Stimulation Components:
1. Negative Control (Grey lid) 28tests
2. TB Antigen, lyophilized (Purple lid)28tests
3. TB Antigen Diluent (Purple lid) 0.9mL
4. Positive Control (Red lid) 28tests
ELISA Components
1. Microplate Strips 12x 8 well
2. Human IFN-γ Standard, lyophilised (Blue lid) 2 x vial
3. Diluent 1 x 40mL
4. Biotin Labeled Antibody (Yellow cap) 1 x 6mL
5. Enzyme Conjugate 100X Concentrate (Brown bottle) 1 x 130μL
6. Wash Buffer 10X Concentrate 1 x 40mL
7. Chromogen Solution A 1 x 6mL
8. Chromogen Solution B 1 x 6mL
9. Stopping Solution 1 x 6mL
Support and Services:
The Elisa Test Kit product technical support and services include:
- Assistance with product installation and initial setup
- Guidance on product usage and troubleshooting
- Product training and education
- Documentation and manuals for reference
- Regular software updates and maintenance
- Remote technical support and assistance
- On-site technical support and repair services
Materials Required But not Provided:
Freshly distilled or deionized water,disposable gloves and timer, appropriate waste containers for potentially contaminated materials, dispensing system and/or pipette, disposable pipette tips, absorbent tissue or clean towel, Water Bath(37±1°C), Plate Reader(dual wavelength 450/600~650nm), microwell aspiration/wash system.
Storage and stability
The components of the kit remain stable 1 year when stored between 2-8°C, Once Stimulation Components are opened, the kit remain stable for 1 week at 2-8℃, 3 weeks at less then -20℃.
Once ELISA Components are opened, the kit remain stable for 3 weeks at 2-8℃.
The dissolved Human IFN-γ Standard should be ready for use.
The dissolved TB Antigen remain stable for 1 week at 2-8℃, 3 weeks at less then -20℃.

 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!