Dengue IgG/IgM 3.0 Rapid Test-Cassette(Serum/Plasma)
High Sensitivity Dengue IgG/IgM Rapid Test Cassette With 24 Months Shelf Life
Product Description:
The Dengue IgG/IgM3.0 Rapid Test is a lateral flow immunoassay for the simultaneous detection and differentiation of IgG anti–dengue virus and IgM anti-dengue virus in human serum or plasma. It is intended to be used by the professionals as a screening test and as an aid in the diagnosis of infection with dengue viruses. Any confirmed with alternative testing method(s).
Applications:
Early diagnosis: This test can help doctors identify dengue virus infection in the early stage of symptom onset so that timely treatment measures can be taken.
Epidemiological survey: In epidemiological surveys, this test can be used to determine whether there is an outbreak of dengue virus infection in a certain area and help monitor the spread of the disease.
Vaccine research: In vaccine research, this test can be used to evaluate the effectiveness and protection of vaccines.
Personal health management: For those who are worried about infection in dengue-endemic areas or during travel, this test can be used as a tool to help them understand their infection status and take appropriate preventive measures.
In general, Dengue IgG/IgM 3.0 Rapid Test can play a role in many aspects, from personal health management to public health response. However, it should be noted that the results of this rapid test may need to be interpreted by professional medical staff and combined with clinical symptoms and other test results to make final diagnosis and treatment decisions.
Support and Services:
The Colloidal Gold Rapid Test is a diagnostic tool designed for the qualitative detection of antibodies to SARS-CoV-2 in human serum, plasma, or whole blood. The test operates on the principle of immunochromatography and can provide results within 15 minutes.
The product comes with the following technical support and services:
- Product training and support for proper use and interpretation of results
- Technical consultation for any questions or concerns
- Product documentation and instructions for use
- Product performance data and validation
Please note that the Colloidal Gold Rapid Test is for professional use only and should not be used as the sole basis for diagnosis or treatment.
Principle of the Assay
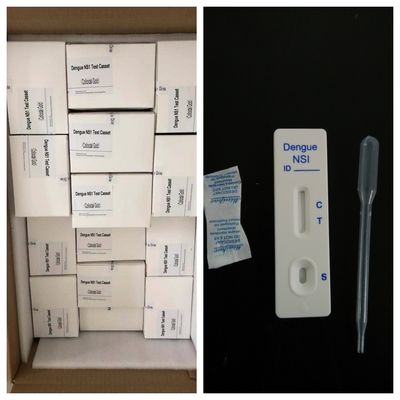
The Dengue IgG/IgM Rapid Test is a lateral flow chromatographic immunoassay. The test cassette consists of: 1) a burgundy
colored conjugate pad containing dengue


recombinant envelope antigens conjugated with colloid gold (dengue conjugates) and rabbit IgG-gold conjugates,2) a nitrocellulose membrane strip containing two test bands
(G and M bands) and a control band (C band).
The G band is pre-coated with the antibody for the detection of IgG anti-dengue virus, M band is coated with antibody for the detection
of IgM anti-dengue virus, and the C band is pre-coated with goat anti rabbit IgG.
When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. IgG anti-dengue virus if present in the specimen will bind to the dengue conjugates. The immunocomplex is then captured by the reagent coated on the G band, forming a burgundy colored G band, indicating a dengue virus IgG positive test result and suggesting a recent or repeat infection.
IgM anti-dengue virus, if present in the specimen, will bind to the dengue conjugates. The immunocomplex is then captured by the reagent pre-coated on the M band, forming a burgundy colored M band, indicating a dengue virus IgM positive test result and suggesting a fresh infection.
Absence of any test bands (G and M) suggests a negative result.
The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti rabbit IgG/rabbit IgG-gold conjugate regardless of the color development on any of the test bands(G and M). Otherwise, the test result is invalid and the specimen must be retested with another device.
Components
1.Each kit contains 40 test devices, each sealed in a foil pouch with two items inside:
a. One cassette device.
b. One desiccant.
2.25 or 30 x 5 µL mini droppers.
3.Sample Diluent (2 bottles, 5 mL).
4.One package insert (instruction for use).
Specimen Collection
Consider any materials of human origin as infectious and handle them using standard biosafety procedures.
Plasma
1. Collect blood specimen into a lavender, blue or green top collection tube (containing EDTA, citrate or heparin, respectively in Vacutainer® ) by veinpuncture.
2. Separate the plasma by centrifugation.
3. Carefully withdraw the plasma into new pre-labeled tube.
Serum
- Collect blood specimen into a red top collection tube (containing no anticoagulants in Vacutainer®) by veinpuncture.
- Allow the blood to clot.
- Separate the serum by centrifugation.
- Carefully withdraw the serum into a new pre-labeled tube.
Test specimens as soon as possible after collecting. Store specimens at 2°C-8°C if not tested immediately.
Store specimens at 2°C-8°C up to 5 days. The specimens should be frozen at -20°C for longer storage.
Avoid multiple freeze-thaw cycles. Prior to testing, bring frozen specimens to room temperature slowly and mix gently. Specimens containing visible particulate matter should be clarified by centrifugation before testing. Do not use samples demonstrating gross lipemia, gross hemolysis or turbidity in order to avoid interference on result interpretation.
| Customization |
Yes |
| Storage |
2-30°C
|
| Shelf life |
24 months
|
| Method |




 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!